This is just amazing. By sending high voltage through a rigid strand of carbon surrounded by a phosphor screen, Ukrainian team has used a field emission electron microscope to directly image s- and p- orbitals. In 10th grade, we built these out of balloons — and quantum mechanics predicted these shapes — but how cool is it to actually see the orbitals?
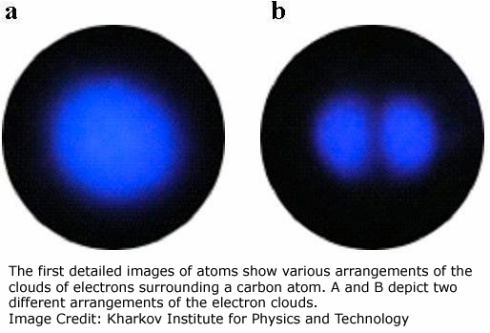
Consider these pictures for a moment. Human thought, determination, and creativity first led to a theory able to explain chemical and physical behaviors, despite having no ability to directly observe the mechanisms involved. Now, 83 years after the Schrödinger equation was first solved, another long chain of determination, stubbornness, and ingenuity has led to something that feels nearly impossible — imaging of a single electron orbital. Awesome.